Background
Successful T-cell based anti-cancer therapies require proliferation and longevity of effector T cells, features that highly depend on metabolic processes. T cells from chronic lymphocytic leukemia (CLL) patients are characterized by acquired T-cell dysfunction, resulting in impaired activation, proliferation and cytotoxicity upon in-vitro activation (van Bruggen et al, Blood 2019). Whilst the role of glucose metabolism in activated T-cells is extensively studied, the contribution of other energy sources is less clear. Lipid metabolism has only recently come into focus as an important source of energy for immune cells.
Aims
Molecular and functional analysis of the lipid metabolic pathway in T cells from CLL patients.
Methods
Peripheral blood mononuclear cells (PBMCs) of untreated CLL patients and, as controls, age-matched healthy donors (HD) were studied. T cells at baseline and after TCR stimulation were analyzed molecularly using transcriptomics and lipidomics, and extensively characterized at protein level by flow cytometry and confocal imaging. Lipid metabolism was manipulated through culturing cells in lipoprotein deficient serum (LPDS), or in the presence of agonists and inhibitors of specific regulatory pathways.
Results
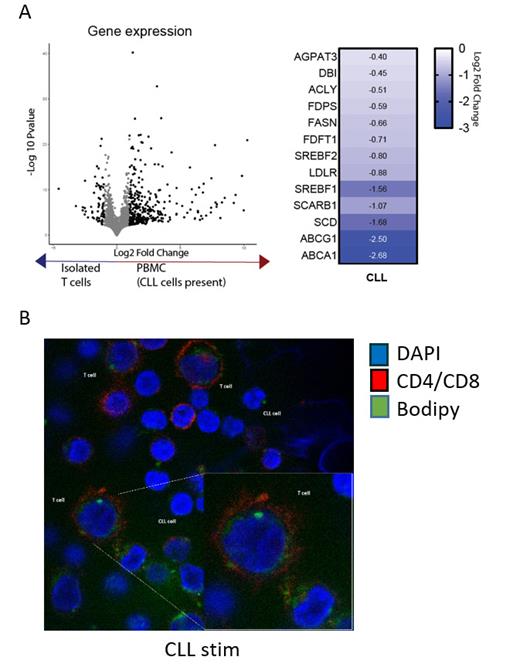
We found the uptake of lipids, specifically cholesterol, during T-cell activation essential for proliferation, which was evidenced by a nearly absent proliferative capacity of HD T cells in the lack of exogenous lipids which was fully rescued by re-addition of cholesterol-rich low-density lipoproteins (LDL). As CLL T cells portray an altered proliferation pattern even when cultured in normal medium, transcriptomic analysis was performed to investigate the expression of key lipid metabolism genes. CLL T-cells had reduced expression of the master transcription factors SREBP1/2, governing lipid and cholesterol metabolism, when stimulated in the presence of their autologous CLL cells (Figure 1A). Accordingly, downstream protein involved in the synthesis and desaturation of lipids and LDL uptake (specifically FASN, LDL-R and SCD) were significantly reduced in CLL compared to HD T cells. Remarkably, despite reduced capacity for LDL uptake, an accumulation of neutral lipids was found in CLL T cells especially upon stimulation (Figure 1B), a feature earlier described in dysfunctional macrophages. The lipid accumulation observed in CLL T cells was supported by the lack of expression of ATGL, a crucial enzyme for the utilization of neutral lipids for energy production and building of new membranes. In line with this, mitochondrial lipid oxidation was decreased in stimulated CLL T cells compared to HD. To investigate the composition of the accumulated lipids, lipidomics analysis was performed, revealing differential abundance of specific lipid classes in stimulated CLL versus HD T cells. Triglycerides were increased while cholesterol esters and phospholipids were reduced in CLL T cells. Triglycerides likely constitute the neutral lipids accumulation found in these cells. As cholesterol esters and phospholipids are essential for proper membrane formation, reduced levels of these species can contribute to the reduced proliferative capacity of CLL T cells.
Summary/conclusion
Altogether, the results presented here demonstrate a disturbed lipid composition and utilization in CLL T-cells, which contributes to T-cell dysfunction on at least two levels: decreased bioenergetics and decreased proliferation. Likewise, lipid deprivation due to both decreased uptake and a misbalanced lipidome leads to altered T-cell function. Therefore, strategies to increase lipid uptake, synthesis and utilization in order to rebalance homeostasis could ameliorate CLL T-cell dysfunction and specifically proliferation-defects in CLL.
Disclosures
Kater:Janssen: Consultancy, Honoraria, Research Funding; Genentech, Inc.: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; LAVA: Consultancy, Honoraria, Research Funding; Astra Zeneca: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding.